Consider the general reversible reaction
aA +bB <-> cC +dD
what is the equilibrium...

Chemistry, 03.11.2020 21:20 Averybloemendaal
Consider the general reversible reaction
aA +bB <-> cC +dD
what is the equilibrium constant expression for the given system?
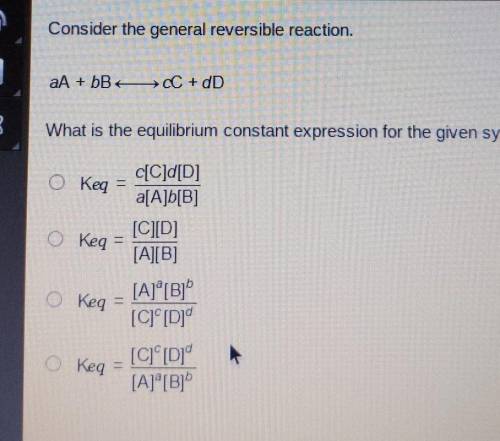

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Questions



History, 16.07.2019 09:20

Social Studies, 16.07.2019 09:20

History, 16.07.2019 09:20

Computers and Technology, 16.07.2019 09:20

Social Studies, 16.07.2019 09:20

Social Studies, 16.07.2019 09:20

Chemistry, 16.07.2019 09:20

Biology, 16.07.2019 09:20


English, 16.07.2019 09:20

Biology, 16.07.2019 09:20

History, 16.07.2019 09:20


Arts, 16.07.2019 09:20






