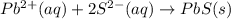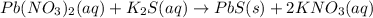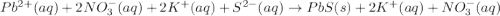Write the net ionic equation (including phases) that corresponds to
pb (no3)2(aq)+k2s(aq)→pbs(...

Chemistry, 28.08.2019 21:00 sarahaziz9526
Write the net ionic equation (including phases) that corresponds to
pb (no3)2(aq)+k2s(aq)→pbs(s)+2kno3(aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions

History, 20.01.2021 21:40

Mathematics, 20.01.2021 21:40

History, 20.01.2021 21:40

Physics, 20.01.2021 21:40

English, 20.01.2021 21:40

Mathematics, 20.01.2021 21:40


Mathematics, 20.01.2021 21:40

Computers and Technology, 20.01.2021 21:40

Arts, 20.01.2021 21:40

Physics, 20.01.2021 21:40


Chemistry, 20.01.2021 21:40



Mathematics, 20.01.2021 21:40


History, 20.01.2021 21:40

History, 20.01.2021 21:40