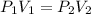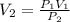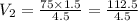Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
A 75.0-ml sample of oxygen has a pressure of 1.50 atm. What will be the new
volume, in milliliters,...
Questions

Mathematics, 23.01.2021 07:30

Chemistry, 23.01.2021 07:30


Mathematics, 23.01.2021 07:30



Mathematics, 23.01.2021 07:30

History, 23.01.2021 07:30

Mathematics, 23.01.2021 07:30



Social Studies, 23.01.2021 07:30

Mathematics, 23.01.2021 07:30



Mathematics, 23.01.2021 07:30