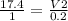Chemistry, 03.11.2020 17:00 diamoniquepowel
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L. The following reaction takes place: N2(g) + 3H2(g)2NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2

Chemistry, 23.06.2019 13:30
Which correctly identifies the parts of a transverse wave? a: crest b: amplitude c: wavelength d: trough a: trough b: amplitude c: crest d: wavelength a: trough b: amplitude c: wavelength d: crest a: crest b: amplitude c: trough d: wavelength
Answers: 1
You know the right answer?
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L...
Questions





Computers and Technology, 28.05.2021 02:40





Mathematics, 28.05.2021 02:40



Mathematics, 28.05.2021 02:40




Mathematics, 28.05.2021 02:40

Mathematics, 28.05.2021 02:40

Social Studies, 28.05.2021 02:40

Social Studies, 28.05.2021 02:40

 →
→