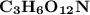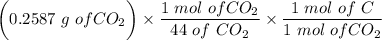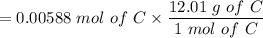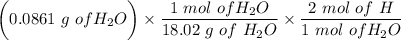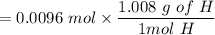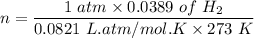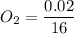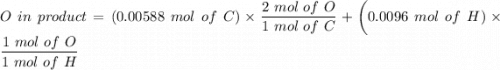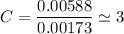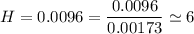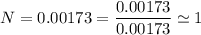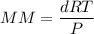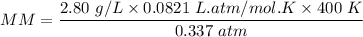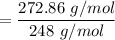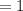An organic compound contains , , , and . Combustion of 0.1023 g of the compound in excess oxygen yielded 0.2587 g and 0.0861 g . A sample of 0.4831 g of the compound was analyzed for nitrogen by the Dumas method. The compound is first reacted by passage over hot : The product gas is then passed through a concentrated solution of to remove the . After passage through the solution, the gas contains and is saturated with water vapor. At STP, 38.9 mL of dry was obtained. In a third experiment, the density of the compound as a gas was found to be 2.86 g/L at 127°C and 256 torr. What are the empirical and molecular formulas of the compound? (Enter the elements in the order: C, H, N, O.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
An organic compound contains , , , and . Combustion of 0.1023 g of the compound in excess oxygen yie...
Questions



Biology, 19.10.2019 07:30













English, 19.10.2019 07:30

Mathematics, 19.10.2019 07:30



Mathematics, 19.10.2019 07:30