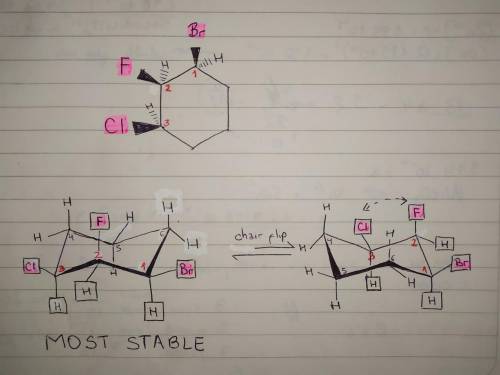
Given the planar trisubstituted cyclohexane, fill in the missing substituents (with H or Cl ) to complete the two possible cyclohexane chair conformations. Then, determine the more stable conformer. You might find it helpful to make a model of the cyclohexane to help visualize the chair conformations. A cyclohexane ring with three substituents, all on wedged bonds. Moving around the ring counter clockwise, there is a bromine on carbon 1, a fluorine on carbon 2 and a chlorine on carbon 3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Given the planar trisubstituted cyclohexane, fill in the missing substituents (with H or Cl ) to com...
Questions





Mathematics, 30.11.2020 18:10





Chemistry, 30.11.2020 18:10





History, 30.11.2020 18:10