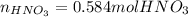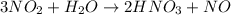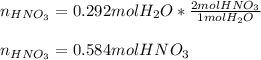Chemistry, 03.11.2020 16:20 zander434556
When nitrogen dioxide (NO2) from car exhaust combines with water in the air, it forms nitric acid (HNO3), which causes acid rain, and nitrogen oxide. Write the balanced chemical equation. How many moles of HNO3 are produced from 0.292 mole of H2O

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
When nitrogen dioxide (NO2) from car exhaust combines with water in the air, it forms nitric acid (H...
Questions

Mathematics, 24.12.2019 21:31

Social Studies, 24.12.2019 21:31

Mathematics, 24.12.2019 21:31

Mathematics, 24.12.2019 21:31

Mathematics, 24.12.2019 21:31


Mathematics, 24.12.2019 21:31

Mathematics, 24.12.2019 21:31


Mathematics, 24.12.2019 21:31







Mathematics, 24.12.2019 21:31

Biology, 24.12.2019 21:31