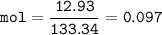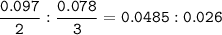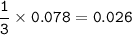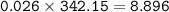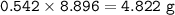Chemistry, 03.11.2020 14:30 krystalhurst97
What mass of Al2(SO4)3 results from mixing 12.93 g of AlCl3 with 10.34 g of (NH4)2SO3 as shown with a 54.2% yield?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
What mass of Al2(SO4)3 results from mixing 12.93 g of AlCl3 with 10.34 g of (NH4)2SO3 as shown with...
Questions

History, 03.12.2021 01:10


Mathematics, 03.12.2021 01:10

Mathematics, 03.12.2021 01:10


English, 03.12.2021 01:10

Mathematics, 03.12.2021 01:10







Geography, 03.12.2021 01:10

Mathematics, 03.12.2021 01:10

History, 03.12.2021 01:10

History, 03.12.2021 01:10



Social Studies, 03.12.2021 01:10