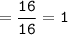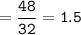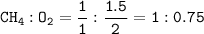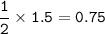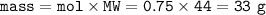Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

You know the right answer?
How many grams of carbon dioxide are produced when 16.0
g of methane and 48.0 g of oxygen gas combu...
Questions

Social Studies, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10



English, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10


Chemistry, 30.04.2021 22:10


Mathematics, 30.04.2021 22:10

Social Studies, 30.04.2021 22:10




Chemistry, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10