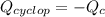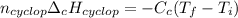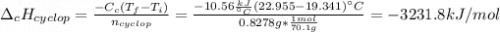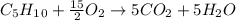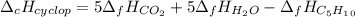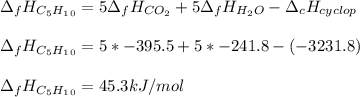Chemistry, 02.11.2020 16:50 uberagentkenny
Cyclopentene is a cyclic hydrocarbon like the ones used in the experiment. In another bomb calorimetry experiment 0.8278 g of cyclopentene is burned and the temperature of the calorimeter increased from 19.341C to 22.955C. The heat capacity of the calorimeter is 10.56 kJ C−1. Calculate the enthalpy of formation of cyclopentene in kJ/mol cyclopentene. Compare this to the accepted value of +32.6 kJ mol−1.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

You know the right answer?
Cyclopentene is a cyclic hydrocarbon like the ones used in the experiment. In another bomb calorimet...
Questions


Health, 18.03.2021 08:30


English, 18.03.2021 08:30

Physics, 18.03.2021 08:30



Arts, 18.03.2021 08:30

Mathematics, 18.03.2021 08:30





English, 18.03.2021 08:30


Mathematics, 18.03.2021 08:30

Mathematics, 18.03.2021 08:30



Mathematics, 18.03.2021 08:40