Chemistry, 02.11.2020 16:40 guzmangisselle
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g) that reacts.
Write a balanced thermochemical equation for the reaction with an energy term in kJ as part of the equation. Note that the answer box for the energy term is case sensitive.
Use the SMALLEST INTEGER coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. If a box is not needed, leave it blank.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g)...
Questions

Health, 13.01.2021 19:00

Mathematics, 13.01.2021 19:00

Mathematics, 13.01.2021 19:00



Mathematics, 13.01.2021 19:00





Mathematics, 13.01.2021 19:00


Mathematics, 13.01.2021 19:00





Computers and Technology, 13.01.2021 19:00

Mathematics, 13.01.2021 19:00

Social Studies, 13.01.2021 19:00

 →
→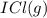
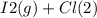 →
→ 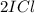
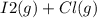 →
→