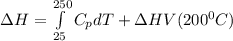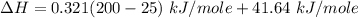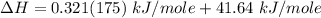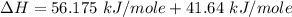Chemistry, 02.11.2020 16:30 monifaWilson
You are writing energy balances for a compound for which you cannot find heat capacity or latent heat data. All you know about the material are its molecular formula (C7H12N) and that it is a liquid at room temperature and has a normal boiling point of 200°C. Use this information to estimate the enthalpy of the vapor of this substance at 200°C relative to the liquid at 25°C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
You are writing energy balances for a compound for which you cannot find heat capacity or latent hea...
Questions






Mathematics, 12.09.2019 19:30














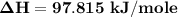
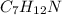
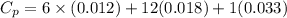
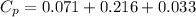
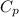 = 0.32 kJ/mole
= 0.32 kJ/mole