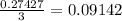Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? (HCl molar...
Questions




History, 02.09.2020 05:01

Mathematics, 02.09.2020 05:01

English, 02.09.2020 05:01

Mathematics, 02.09.2020 05:01

Biology, 02.09.2020 05:01


Mathematics, 02.09.2020 05:01



Computers and Technology, 02.09.2020 05:01

Computers and Technology, 02.09.2020 05:01

Mathematics, 02.09.2020 05:01





Mathematics, 02.09.2020 05:01

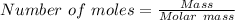
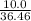
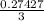 mole of BCl₃ would be needed to produce 0.27427 mole of HCl
mole of BCl₃ would be needed to produce 0.27427 mole of HCl