The kinetic-molecular theory states that ideal gas molecules do which of
the following?*
are...

Chemistry, 02.11.2020 14:00 katwilf1771
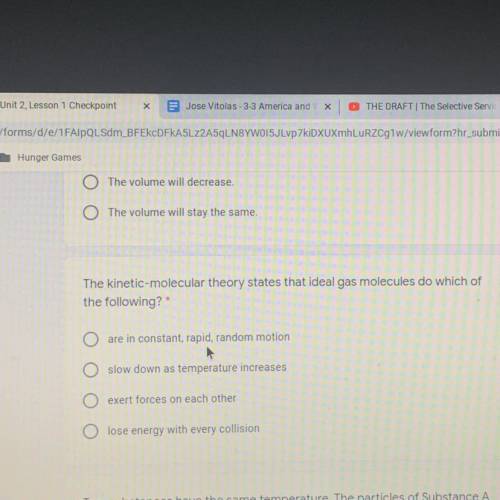
The kinetic-molecular theory states that ideal gas molecules do which of
the following?*
are in constant, rapid, random motion
O slow down as temperature increases
O exert forces on each other
O lose energy with every collision

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

You know the right answer?
Questions

Mathematics, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31







Mathematics, 24.01.2020 03:31












