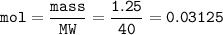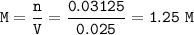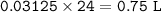Tasks
5) 1.25 g of calcium is added to 25 cm3 of water. Calculate the
concentration of the ca...


Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Questions

Mathematics, 22.04.2021 22:30






Geography, 22.04.2021 22:30


Mathematics, 22.04.2021 22:30



Mathematics, 22.04.2021 22:30


English, 22.04.2021 22:30

Mathematics, 22.04.2021 22:30

Mathematics, 22.04.2021 22:30