
Chemistry, 02.11.2020 05:50 ghari112345
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00 moles of potassium chlorate decomposes?
a. 134 L
b. 67.2 L
c. 22.4 L
d. 11.2 L
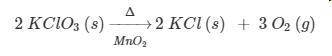

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

You know the right answer?
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00...
Questions



Mathematics, 10.03.2020 07:48






Mathematics, 10.03.2020 07:48





Computers and Technology, 10.03.2020 07:48








