Calculate the energy of a photon of light with
a frequency of 5.109 × 1016 Hz....

Chemistry, 02.11.2020 02:10 lareynademividp0a99r
Calculate the energy of a photon of light with
a frequency of 5.109 × 1016 Hz.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Questions


Mathematics, 16.10.2020 17:01

Geography, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01


Spanish, 16.10.2020 17:01

Chemistry, 16.10.2020 17:01


History, 16.10.2020 17:01


Spanish, 16.10.2020 17:01

English, 16.10.2020 17:01


Chemistry, 16.10.2020 17:01

Social Studies, 16.10.2020 17:01

Advanced Placement (AP), 16.10.2020 17:01

Mathematics, 16.10.2020 17:01



History, 16.10.2020 17:01

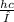 , where h is Planck's Constant,c is the speed of light, and λ is the wavelength of the photon.
, where h is Planck's Constant,c is the speed of light, and λ is the wavelength of the photon.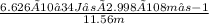 = 1.718×10−26J.
= 1.718×10−26J.

