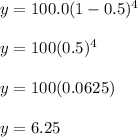Chemistry, 01.11.2020 08:20 cfigueroablan
The half-life of carbon-14 is 5,730 years. If
you started with 100.0 g of carbon-14, how
much would remain after 4 half-lives?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
The half-life of carbon-14 is 5,730 years. If
you started with 100.0 g of carbon-14, how
Questions

Chemistry, 17.11.2020 22:00



Mathematics, 17.11.2020 22:00







History, 17.11.2020 22:00


English, 17.11.2020 22:00

Computers and Technology, 17.11.2020 22:00

Biology, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:10

Mathematics, 17.11.2020 22:10

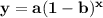 where a is the initial amount of the substance, b is the decay factor, and x is the time elapsed (this is also known as the exponential decay function). We can define our values and substitute them into the equation.
where a is the initial amount of the substance, b is the decay factor, and x is the time elapsed (this is also known as the exponential decay function). We can define our values and substitute them into the equation.