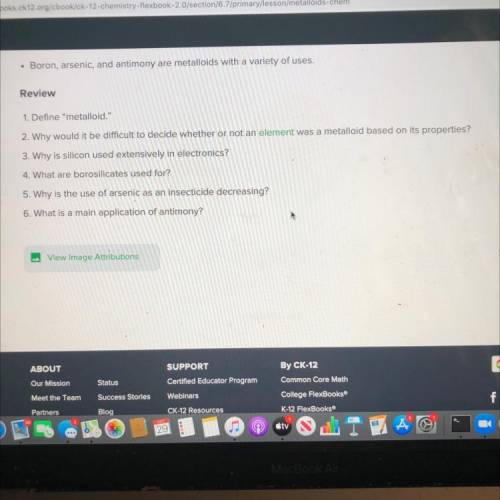
Please help me I need these answers
...

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 12:30
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
You know the right answer?
Questions


Mathematics, 05.02.2021 01:20



English, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20

English, 05.02.2021 01:20

Chemistry, 05.02.2021 01:20

Computers and Technology, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


Social Studies, 05.02.2021 01:20

Biology, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


Mathematics, 05.02.2021 01:20

French, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


History, 05.02.2021 01:20