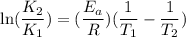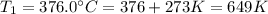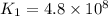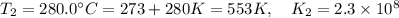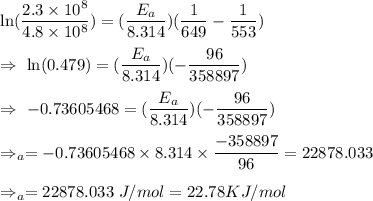The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
376.0°C 4.8 x 10^8
280°C 2.3 x 10^8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy for this reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
Questions

Mathematics, 16.10.2020 19:01


Mathematics, 16.10.2020 19:01


Mathematics, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01



Mathematics, 16.10.2020 19:01

Advanced Placement (AP), 16.10.2020 19:01

Mathematics, 16.10.2020 19:01


Computers and Technology, 16.10.2020 19:01

English, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01

English, 16.10.2020 19:01



English, 16.10.2020 19:01

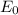 follows by formula:
follows by formula: