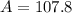Copying is not allowed. You must show all work in order to receive credit. Naturally occurring silver has two isotopes. Isotope A has a relative mass of 106.9051 and an abundance of 51.82%. Isotope B has a relative mass of 108.9047. Calculate the atomic mass of silver from these data.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
Copying is not allowed. You must show all work in order to receive credit. Naturally occurring silve...
Questions


Mathematics, 27.04.2021 19:40


English, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

Geography, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40

History, 27.04.2021 19:40

Physics, 27.04.2021 19:40

Mathematics, 27.04.2021 19:40




Mathematics, 27.04.2021 19:40



Mathematics, 27.04.2021 19:40

English, 27.04.2021 19:40


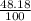
![A=\sum[(106.9051)\times \frac{51.82}{100})+(108.9047)\times \frac{48.18}{100}]]](/tpl/images/0851/2823/92a78.png)