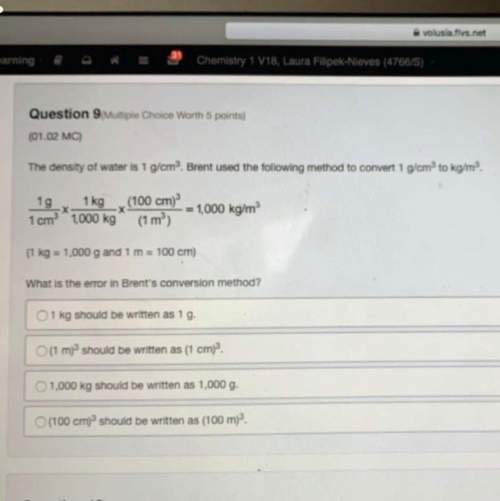
Chemistry, 28.10.2020 21:10 Rubendelarosa1529
Among the elements of the main group, the general trend in the first ionization energy moving across a period is not followed between group 2 and group 13.
Which best explains these exceptions?
The ionization energy decreases because the full s orbital shields the electron entering the p orbital.
The ionization energy increases because the full s orbital shields the electron entering the p orbital.
The ionization energy decreases because the stability of the half-full p subshell is increased.
The ionization energy increases because the stability of the half-full p subshell is increased.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Among the elements of the main group, the general trend in the first ionization energy moving across...
Questions


History, 12.11.2020 02:50

Mathematics, 12.11.2020 02:50







German, 12.11.2020 02:50

Mathematics, 12.11.2020 02:50








English, 12.11.2020 02:50