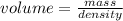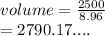Chemistry, 28.10.2020 19:00 heberavilesramirez52
Copper is known to have a density of 8.96g/cm3. If I have a 25 kg block of copper, how much volume does it take up in cm3? In Liters?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Copper is known to have a density of 8.96g/cm3. If I have a 25 kg block of copper,
how much volume...
Questions

Physics, 25.09.2020 08:01

History, 25.09.2020 08:01

Biology, 25.09.2020 08:01



Mathematics, 25.09.2020 08:01


Mathematics, 25.09.2020 08:01


Geography, 25.09.2020 08:01



Mathematics, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01

Engineering, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01



Mathematics, 25.09.2020 08:01