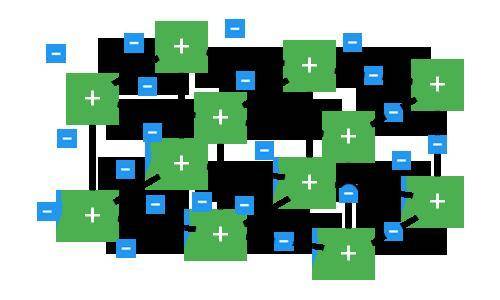
Which type of compound is represented in the model and why?
Select all that apply.
A. T...

Which type of compound is represented in the model and why?
Select all that apply.
A. This model is not a metallic compound because the electrons are being transferred between different ions in the crystal structure.
B. This model is an ionic compound because the rigid formation is an example of a crystal lattice.
C. This model is a metallic compound because it consists of free-moving electrons in a crystal formation.
D. This model is not an ionic compound because the electrons are not being transferred between positive and negative ions; instead, only positive ions are present.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1



Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Questions

Mathematics, 21.12.2019 09:31



English, 21.12.2019 10:31


Social Studies, 21.12.2019 10:31


Social Studies, 21.12.2019 10:31

Mathematics, 21.12.2019 10:31



History, 21.12.2019 10:31

History, 21.12.2019 10:31


History, 21.12.2019 10:31







