The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag....

Chemistry, 28.10.2020 14:00 trevorhenyan51
The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag. An atom of Ag will have a certain ground
state electron configuration according to the
Aufbau principle. The outermost electrons in
Ag will have the configuration
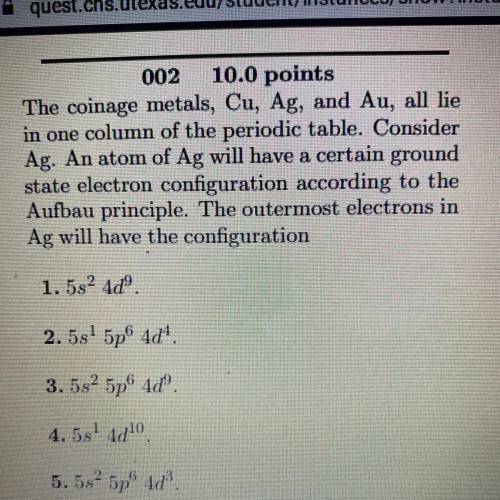

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
Questions

Computers and Technology, 14.06.2021 18:20



Mathematics, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20

Arts, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20


Physics, 14.06.2021 18:20

Mathematics, 14.06.2021 18:20

History, 14.06.2021 18:20


World Languages, 14.06.2021 18:20






