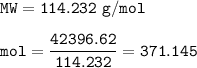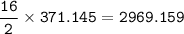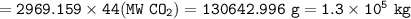Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
What mass of CO2 (in kilograms) does the combustion of a 16-gallon tank of gasoline release into the...
Questions

Mathematics, 10.12.2019 10:31

Chemistry, 10.12.2019 10:31

Chemistry, 10.12.2019 10:31






Mathematics, 10.12.2019 10:31




Social Studies, 10.12.2019 10:31

History, 10.12.2019 10:31

English, 10.12.2019 10:31



Biology, 10.12.2019 10:31

Biology, 10.12.2019 10:31

Computers and Technology, 10.12.2019 10:31