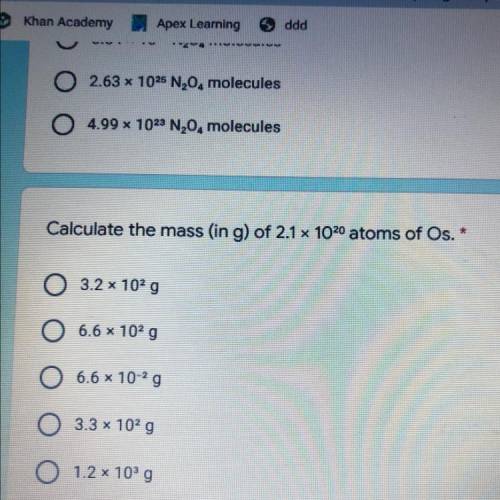
Calculate the mass (in g) of 2.1 x 1020 atoms of Os.
...

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Questions

Business, 22.07.2019 22:00



Biology, 22.07.2019 22:00









History, 22.07.2019 22:00



Arts, 22.07.2019 22:00


Mathematics, 22.07.2019 22:00

History, 22.07.2019 22:00