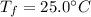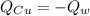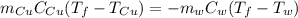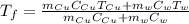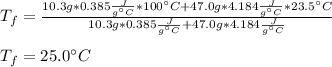An insulated container is used to hold 47.0 g of water at 23.5°C. A sample of copper weighing 10.3 g is placed in a dry test tube and
heated for 30 minutes in a boiling water bath at 100.0°C. The heated test tube is carefully removed from the water bath with laboratory
tongs and inclined so that the copper slides into the water in the insulated container. Given that the specific heat of solid copper is
0.385 J/(g.°C), calculate the maximum temperature of the water in the insulated container after the copper metal is added.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

You know the right answer?
An insulated container is used to hold 47.0 g of water at 23.5°C. A sample of copper weighing 10.3 g...
Questions

Spanish, 28.03.2021 19:00

Medicine, 28.03.2021 19:00

Mathematics, 28.03.2021 19:00



Computers and Technology, 28.03.2021 19:00


Physics, 28.03.2021 19:10

SAT, 28.03.2021 19:10

Mathematics, 28.03.2021 19:10


Business, 28.03.2021 19:10

Mathematics, 28.03.2021 19:10

Computers and Technology, 28.03.2021 19:10


History, 28.03.2021 19:10



English, 28.03.2021 19:10

Mathematics, 28.03.2021 19:10