Chemistry, 17.09.2019 04:30 kyliegriffis
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
which ion(s) would not be spectator ions?
a) pb2+ , no3-
b) k+ , i
c) no3- , pb2+
d) pb2+, i –
e) all the above ions are in the net ionic equation

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 3
You know the right answer?
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
Questions

Mathematics, 05.11.2020 01:00



Chemistry, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00




English, 05.11.2020 01:00




Mathematics, 05.11.2020 01:00



History, 05.11.2020 01:00



English, 05.11.2020 01:00

Advanced Placement (AP), 05.11.2020 01:00

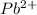 and
and 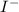
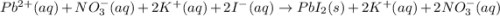
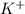 and
and 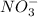 and are spectator ions.
and are spectator ions.