
Chemistry, 27.10.2020 17:20 mvongphakdy8124
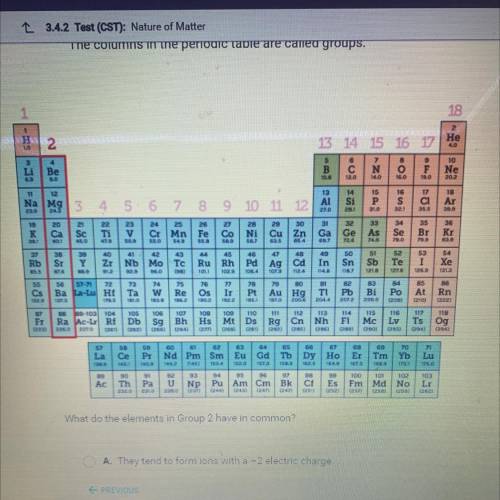
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge.
B. They tend to form ions with a +2 electric charge.
C. They are all highly reactive nonmetals.
D. They are all found in nature in their pure forms.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1


Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge...
Questions










Mathematics, 11.01.2020 07:31

English, 11.01.2020 07:31


Health, 11.01.2020 07:31





Mathematics, 11.01.2020 07:31

History, 11.01.2020 07:31



