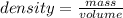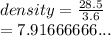Chemistry, 27.10.2020 17:20 Ghhkgu5120
28.5 g of iron shot is added to a graduated cylinder contains 45.50 ml pf water. The water level rises to the 49.10 ml mark from this information calculate the denisty of iron

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 23.06.2019 15:00
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1

Chemistry, 23.06.2019 19:30
The total amount of fresh water on earth is estimated to be 3.73 x 10^8 km^3. what is this volume in cubic meters? in cubic feet?
Answers: 1
You know the right answer?
28.5 g of iron shot is added to a graduated cylinder contains 45.50 ml pf water. The water level ris...
Questions


English, 08.11.2019 13:31

History, 08.11.2019 13:31

Social Studies, 08.11.2019 13:31



English, 08.11.2019 13:31

Arts, 08.11.2019 13:31

Biology, 08.11.2019 13:31



Chemistry, 08.11.2019 13:31

Biology, 08.11.2019 13:31


Mathematics, 08.11.2019 13:31



Mathematics, 08.11.2019 13:31

Social Studies, 08.11.2019 13:31