
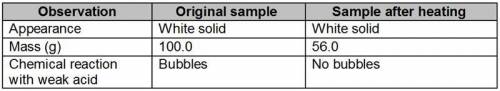
Obi is trying to determine if a powdery, solid substance is an element or a compound. After recording some observations, he strongly heats a sample of the solid over a burner flame. After fifteen minutes, he turns off the flame and allows the sample to cool. He records his final observations in the data table.
Which is the best explanation of his results?
The heating changed some of the sample to gas, causing the mass to decrease without breaking down the sample. Therefore, the original sample is a compound.
The appearance stayed the same, showing that the sample was not broken down by heating. Therefore, the original substance is an element.
The mass decreased during heating and some of the impurities escaped. Therefore, the original substance is an element.
The chemical reaction with acid changed, showing that the sample was broken down by heating. Therefore, the original substance is a compound

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Obi is trying to determine if a powdery, solid substance is an element or a compound. After recordin...
Questions

Social Studies, 28.09.2019 13:10



English, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10

Spanish, 28.09.2019 13:10

English, 28.09.2019 13:10


English, 28.09.2019 13:10

History, 28.09.2019 13:10

Biology, 28.09.2019 13:10

Health, 28.09.2019 13:10






Social Studies, 28.09.2019 13:10




