
Chemistry, 27.10.2020 16:00 emojigirl5754
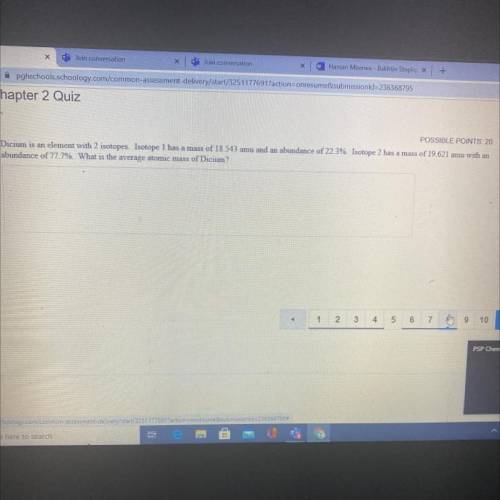
Dicium is an element with 2 isotopes. Isotope 1 has a mass of 18.543 amu and an abundance of 22.3%. Isotopes 2 has a mass of 19.621 amu with an abundance of 77.7% what is the average atomic mass dicium

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1



Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Dicium is an element with 2 isotopes. Isotope 1 has a mass of 18.543 amu and an abundance of 22.3%....
Questions


Mathematics, 17.12.2019 09:31

Mathematics, 17.12.2019 09:31



Arts, 17.12.2019 09:31

Mathematics, 17.12.2019 09:31





Geography, 17.12.2019 09:31

Computers and Technology, 17.12.2019 09:31



English, 17.12.2019 09:31



History, 17.12.2019 09:31



