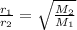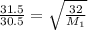Chemistry, 27.10.2020 04:30 andrew5632
16. The diffusion rate of an unknown gas is measured to be 31.50 mL/min. Under
identical conditions, the diffusion rate of oxygen gas is measured to be 30.50
mL/min. Hint: diffusion rate is directly related to velocity.
Determine the identity of the unknown gas from the following options:
a. CH4
b. CO
C. NO
d. CO2
e. NO2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2


Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
16. The diffusion rate of an unknown gas is measured to be 31.50 mL/min. Under
identical conditions...
Questions

Chemistry, 22.09.2019 00:20

History, 22.09.2019 00:20



Mathematics, 22.09.2019 00:20

Business, 22.09.2019 00:20



Mathematics, 22.09.2019 00:20

Physics, 22.09.2019 00:20

Biology, 22.09.2019 00:20


History, 22.09.2019 00:20

Mathematics, 22.09.2019 00:20





History, 22.09.2019 00:20

Biology, 22.09.2019 00:20