Calculate the solubility of solid CaF2 (Ksp =
in a 0.025 M NaF solution...

Chemistry, 26.10.2020 20:00 hannamcbrayer1
Calculate the solubility of solid CaF2 (Ksp =
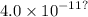
in a 0.025 M NaF solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Questions

Biology, 08.10.2019 22:30

Mathematics, 08.10.2019 22:30

Mathematics, 08.10.2019 22:30

Health, 08.10.2019 22:30


History, 08.10.2019 22:30




Physics, 08.10.2019 22:30



Chemistry, 08.10.2019 22:30




Mathematics, 08.10.2019 22:30






