
Chemistry, 26.10.2020 19:40 Buttercream16
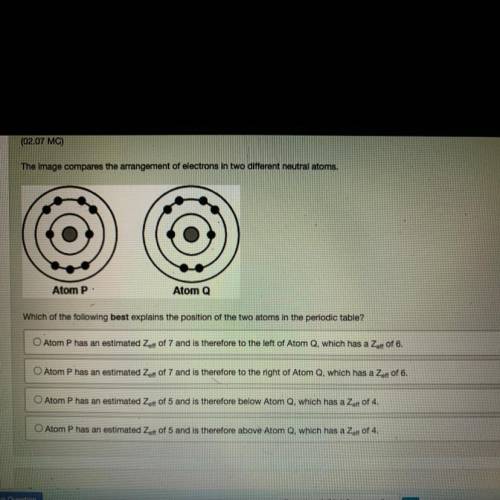
The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom Q
Which of the following best explains the position of the two atoms in the periodic table?
O Atom P has an estimated Zaff of 7 and is therefore to the left of Atom Q, which has a Zert of 6.
O Atom P has an estimated Zeft of 7 and is therefore to the right of Atom Q, which has a Zer of 6.
O Atom P has an estimated Zert of 5 and is therefore below Atom Q, which has a Zoff of 4.
O Atom P has an estimated Zoff of 5 and is therefore above Atom Q, which has a Zet of 4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom...
Atom...
Questions




Social Studies, 29.01.2022 14:00





Chemistry, 29.01.2022 14:00








Computers and Technology, 29.01.2022 14:00


Mathematics, 29.01.2022 14:00

Biology, 29.01.2022 14:00



