
Chemistry, 26.10.2020 17:20 justinchou814
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H is 1.00783 amuamu, and the mass of a neutron is 1.00866 amuamu .)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H i...
Questions



Physics, 14.11.2020 05:00



Mathematics, 14.11.2020 05:00


Biology, 14.11.2020 05:00


Mathematics, 14.11.2020 05:00


Mathematics, 14.11.2020 05:00


Social Studies, 14.11.2020 05:00

Advanced Placement (AP), 14.11.2020 05:10

Social Studies, 14.11.2020 05:10

Mathematics, 14.11.2020 05:10

Mathematics, 14.11.2020 05:10

English, 14.11.2020 05:10

Physics, 14.11.2020 05:10

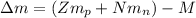
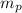 : is the proton mass = 1.00783 amu
: is the proton mass = 1.00783 amu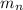 : is the neutron mass = 1.00866 amu
: is the neutron mass = 1.00866 amu ![\Delta m = (Zm_{p} + Nm_{n}) - M = [92*1.00783 amu + (239 - 92)*1.00866 amu] - 239.05429 amu = 1.93909 amu](/tpl/images/0840/4283/ccb79.png)


