
Chemistry, 26.10.2020 17:00 datboyjulio21
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What is the limiting reagent in the reaction and what is the percent theoretical yield?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What i...
Questions


Physics, 12.12.2020 22:10


Mathematics, 12.12.2020 22:10

Mathematics, 12.12.2020 22:10



Chemistry, 12.12.2020 22:10

Arts, 12.12.2020 22:10

Mathematics, 12.12.2020 22:10

Mathematics, 12.12.2020 22:10


Mathematics, 12.12.2020 22:10


Mathematics, 12.12.2020 22:10

Mathematics, 12.12.2020 22:10

Physics, 12.12.2020 22:10


Computers and Technology, 12.12.2020 22:10

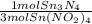 = 0.112 mol Sn₃N₄
= 0.112 mol Sn₃N₄

