
Chemistry, 26.10.2020 16:40 jsmith4184
What molar ratio of acetic acid to sodium acetate is required to create a buffer solution having a pH of 4.93 at 25°C? Ka for HC2H3O2 is 1.8 × 10–5.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
What molar ratio of acetic acid to sodium acetate is required to create a buffer solution having a p...
Questions

History, 02.10.2020 21:01



Mathematics, 02.10.2020 21:01

Geography, 02.10.2020 21:01





Biology, 02.10.2020 21:01

English, 02.10.2020 21:01



Advanced Placement (AP), 02.10.2020 21:01


History, 02.10.2020 21:01

Social Studies, 02.10.2020 21:01


Spanish, 02.10.2020 21:01

Chemistry, 02.10.2020 21:01

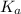 = Acid ionization constant of acetic acid =
= Acid ionization constant of acetic acid = 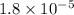
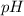 = Potential of hydrogen of buffer solution =
= Potential of hydrogen of buffer solution = 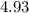
![K_a=\dfrac{[H+][A-]}{[HA]}\\\Rightarrow K_a=[H+]\dfrac{[A-]}{[HA]}](/tpl/images/0840/1638/61c39.png)
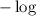 on both sides of the equation we get
on both sides of the equation we get![-\log K_a=-\log [H+]-\log\dfrac{[A-]}{[HA]}\\\Rightarrow pK_a=pH-\log\dfrac{[A-]}{[HA]}\\\Rightarrow pH=pK_a+\log\dfrac{[A-]}{[HA]}](/tpl/images/0840/1638/8d914.png)
![4.93=-\log(1.8\times 10^{-5})+\log\dfrac{[A-]}{[HA]}\\\Rightarrow 4.93-4.74=\log\dfrac{[A-]}{[HA]}\\\Rightarrow 0.19=\log\dfrac{[A-]}{[HA]}\\\Rightarrow 1.55=\dfrac{[A-]}{[HA]}\\\Rightarrow \dfrac{[HA]}{[A-]}=0.65](/tpl/images/0840/1638/a0e07.png)


