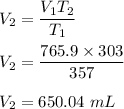Chemistry, 26.10.2020 16:40 androw4116
A sample of oxygen gas initially at 303 K was heated to 357 K. If the volume of the oxygen gas sample at 357 K is 765.9 mL, what was its volume at 303 K? V= mL

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
A sample of oxygen gas initially at 303 K was heated to 357 K. If the volume of the oxygen gas sampl...
Questions







Mathematics, 15.04.2020 03:04







Biology, 15.04.2020 03:04


Biology, 15.04.2020 03:04




Mathematics, 15.04.2020 03:04