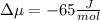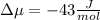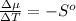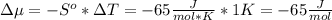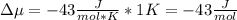Chemistry, 26.10.2020 16:40 corey36dylon
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the same temperature is 43 J K−1 mol−1. Calculate the change in chemical potential of liquid water and of ice when the temperature is increased by 1 K from the normal melting point. Giving your reasons, explain which phase is thermodynamically the more stable at the new temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

You know the right answer?
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the sam...
Questions

Biology, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01



Mathematics, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01

English, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01


Spanish, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01


Spanish, 20.10.2020 04:01


Spanish, 20.10.2020 04:01


History, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01