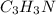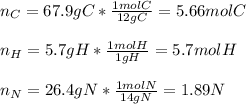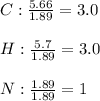Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Calculate the empirical formula of a compound that contains 67.9% carbon, 5.7 % H and 26.4 % N. When...
Questions

Mathematics, 08.09.2021 21:10

Computers and Technology, 08.09.2021 21:10


Mathematics, 08.09.2021 21:10


Computers and Technology, 08.09.2021 21:10


English, 08.09.2021 21:10

Health, 08.09.2021 21:10




Mathematics, 08.09.2021 21:10



Spanish, 08.09.2021 21:10

English, 08.09.2021 21:10


Biology, 08.09.2021 21:20

Business, 08.09.2021 21:20