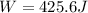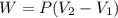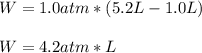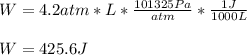Chemistry, 26.10.2020 16:40 lucygperez4099
Calculate the work for the expansion of CO2 from 1.0 to 5.2 liters against a pressure of 1.0 atm at constant temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
Calculate the work for the expansion of CO2 from 1.0 to 5.2 liters against a pressure of 1.0 atm at...
Questions



Mathematics, 26.10.2019 01:43


English, 26.10.2019 01:43

Mathematics, 26.10.2019 01:43