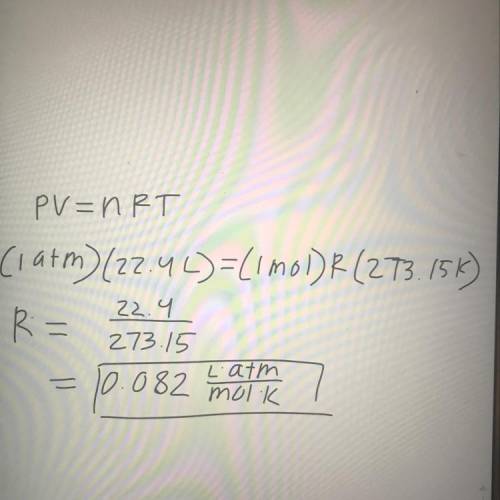Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
Given that one mole occupies 22.4 L at STP, show that R = .082 (atm * L)/(mol*K)...
Questions




Mathematics, 04.09.2020 05:01

Physics, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01

Biology, 04.09.2020 05:01


History, 04.09.2020 05:01

Advanced Placement (AP), 04.09.2020 05:01


Mathematics, 04.09.2020 05:01




Mathematics, 04.09.2020 05:01