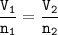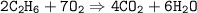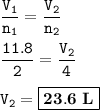Chemistry, 25.10.2020 19:30 AriaMartinez
What volume (in L) of carbon dioxide will be produced from the reaction of 11.8L of ethane

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
What volume (in L) of carbon dioxide will be produced from the reaction of 11.8L of ethane...
Questions

Mathematics, 30.03.2021 18:00



Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00


History, 30.03.2021 18:00

Business, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00





Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

Mathematics, 30.03.2021 18:00

English, 30.03.2021 18:00

Social Studies, 30.03.2021 18:00