

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
- Using the choices below, which is the complete symbol for the isotope that has the following subat...
Questions

SAT, 14.12.2020 03:40

History, 14.12.2020 03:40



Mathematics, 14.12.2020 03:40

Mathematics, 14.12.2020 03:40



Mathematics, 14.12.2020 03:40




English, 14.12.2020 03:40




Mathematics, 14.12.2020 03:40



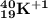 Further explanation
Further explanation



