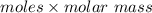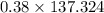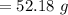Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
A 12.39 g sample of phosphorus reacts with 40.75 g of chlorine to form only phosphorus trichloride (...
Questions

Biology, 24.03.2020 04:59


Computers and Technology, 24.03.2020 04:59

English, 24.03.2020 04:59

Business, 24.03.2020 04:59

English, 24.03.2020 04:59

Mathematics, 24.03.2020 04:59

Mathematics, 24.03.2020 04:59

Biology, 24.03.2020 04:59

Spanish, 24.03.2020 04:59




Mathematics, 24.03.2020 05:00





Mathematics, 24.03.2020 05:00


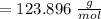
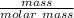
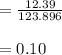
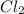 molar mass
molar mass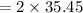
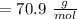
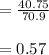
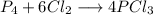
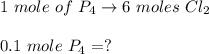
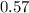 ,
, 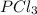 Theoretical performance
Theoretical performance