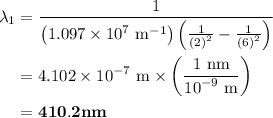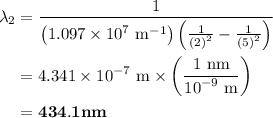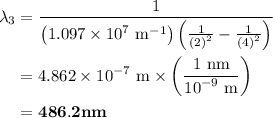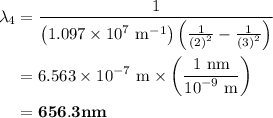Chemistry, 17.09.2019 12:20 lizzyhearts
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2 principle quantum level. calculate the wavelengths associated with the spectral transitions of the hydrogen atom from the n=6,5,4 and 3 to the n=2 level.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2...
Questions

Mathematics, 04.03.2021 19:20


Mathematics, 04.03.2021 19:20




World Languages, 04.03.2021 19:20


Mathematics, 04.03.2021 19:20




History, 04.03.2021 19:20

Mathematics, 04.03.2021 19:20


Mathematics, 04.03.2021 19:20




Spanish, 04.03.2021 19:20

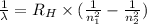
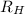 = Rydberg constant =
= Rydberg constant = 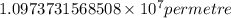
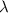 = wavelength
= wavelength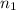 and
and 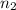 are the level of transitions.
are the level of transitions.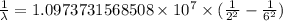
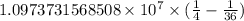
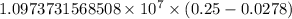
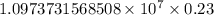
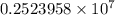
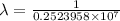


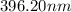
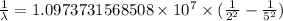
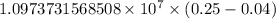
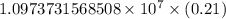
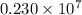
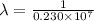


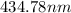
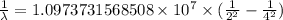
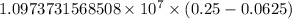
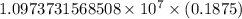
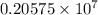
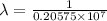


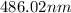
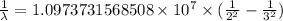
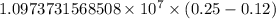
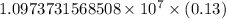
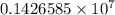
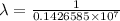


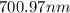
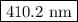 .
.
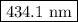 .
.
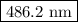 .
.
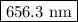 .
.
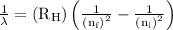 …… (1)
…… (1)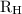 is the Rydberg constant that has the value
is the Rydberg constant that has the value 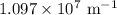 ,
, 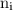 is the initial energy level of transition, and
is the initial energy level of transition, and 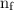 is the final energy level of transition.
is the final energy level of transition.
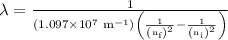 …… (2)
…… (2)