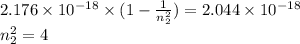Chemistry, 22.10.2020 19:01 jak000067oyyfia
Determine the end (final) value of n in a hydrogen atom transition, if the electron starts in n = 1 and the atom absorbs a photon of light with an energy of 2.044 × 10-18 J.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Determine the end (final) value of n in a hydrogen atom transition, if the electron starts in n = 1...
Questions




Spanish, 08.04.2021 20:10




Mathematics, 08.04.2021 20:10



Biology, 08.04.2021 20:10



Geography, 08.04.2021 20:10


Mathematics, 08.04.2021 20:10


Biology, 08.04.2021 20:20


Mathematics, 08.04.2021 20:20