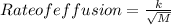Chemistry, 22.10.2020 19:01 juliannabartra
At constant temperature, He effuses 6.04 times faster than a gas having an unknown molar mass. Determine the molar mass of the unknown gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
At constant temperature, He effuses 6.04 times faster than a gas having an unknown molar mass. Deter...
Questions

Mathematics, 13.09.2020 02:01

English, 13.09.2020 02:01

Biology, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

History, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

English, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01